|
Lactic Acid
Lactic acid (2-hydroxypropanoic acid) was discovered and
isolated in 1780 by the Swedish chemist Scheele in sour milk.
It was first commercially produced in USA in 1881. Its early
utilization was in the leather and textile industries. Lactic
acid is widely used in the food industry as an acidulant,
preservative, precursor for stearoyl-2-lactylates. Perhaps its
greatest industrial potential is for biodegradable polymers such
as polylactic acid. Lactic acid can be produced by chemical
synthesis or by fermentation. Our research program on lactic
acid began in the early 1980s and focused on two major areas:
- Bioreactor design to improve productivity of the
fermentation
- Downstream processing to recover lactic acid.
BIOREACTOR DESIGN
Based on past success with membrane bioreactors, we started work in 1983 on continuous
production of lactic acid. We designed a continuous membrane bioreactor (CMB) as a
continuous stirred tank reactor (CSTR) coupled in a semi
closed loop configuration to a membrane module, as shown in the
diagram below.
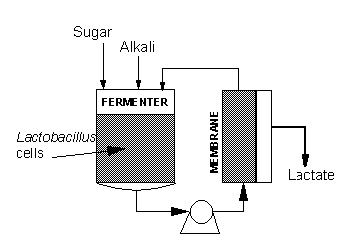
Synthetic semi-permeable membranes are used to separate and
recycle the lactic acid bacteria, while simultaneously removing
the lactate as it is formed. This has several advantages over
batch fermenters: - The continuous separation and
recovery of the bacterial cells will reduce cycle time of the
fermenters, since there will be little or no time lost due to
start-up and shut down as in present batch fermenters.
- The recycle of the cells will allow us to obtain much
higher cell densities than currently practiced. Laboratory
studies have shown a 100-fold increase in cell numbers in the
CMB during operation. The high concentration allows us to pump
the feedstock through the fermenters much faster.
- "Cell wash-out" is eliminated, thereby
allowing operation at dilution rates greater than the specific
growth rate of the organism.
- The continuous removal of lactate allows us to maintain
the fermenter at just below the lactate level which inactivates
the cells. Thus the cells are always viable and producing
lactate.

The graph illustrates the
improvements to be expected with membrane-based fermenters. In
laboratory trials, productivities 10-20 times higher than batch
fermenters have been obtained. Other benefits have been
observed: - The membrane bioreactors are very flexible,
allowing a range of outputs that can be matched very easily to
the demands of upstream and downstream operations.
- The product stream from the fermenter is clear,
containing no suspended matter. This will improve the subsequent
recovery and purification process, with a further reduction in
cost.
- The membrane units are available in modular systems,
making expansion easy.
- Due to the high productivity, floor space requirements
for the membrane bioreactor system are much less than with
present-day batch fermenters.
Another configuration we investigated was the hollow fiber
bioreactor, which was operated in a quasi plug flow mode. The
CSTR-membrane configuration is preferred since it is a
well-mixed system that allows us to efficiently neutralize the
fermentation broth with the appropriate alkali (usually ammonium
or sodium hydroxide).
DOWNSTREAM PROCESSING
Lactic acid can be separated and substantially purified from
fermentation broths by several membrane-based unit operations as
shown in the diagram below:
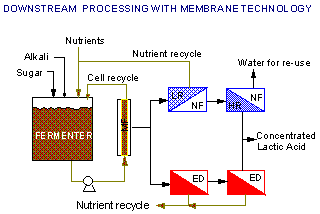
- Microfiltration or ultrafiltration for cell
separation and recycle
- Nanofiltration for separation of the lactic acid from
other broth components using low rejection (LR) membranes
- Concentrating the lactate using reverse osmosis (RO) or
a combination of high rejection (HR) and low rejection (LR)
nanofiltration membranes
- Electrodialysis (ED) for simultaneous separation and
concentration of lactate. A conventional anion-/cation-exchange
membrane ED system will purify and concentrate the lactate, but
the lactate product will still be in the salt form (if the salt
form was produced in the fermentation). On the other hand, a
bipolar membrane ED system will result in the acid form of
lactic acid and allow the recycle of the alkali used for
neutralizing the fermentation broth. This minimizes alkali cost,
as well as eliminating the waste product (e.g., calcium sulfate)
generated in conventional downstream processes for organic
acids.
 Publications on this topic Publications on this topic
 Research -- Other topics Research -- Other topics
Updated January 2006 by mcheryan@uiuc.edu
|



 Publications on this topic
Publications on this topic