|
Dextrose (glucose) is the primary feedstock for numerous products, including corn sweeteners, fuel ethanol and organic acids (e.g., lactic acid, acetic acid, citric acid). It is produced by enzyme hydrolysis of starch (e.g., from corn) or cellulose (e.g., from woody biomass). As practiced today, the saccharification step has many limitations:
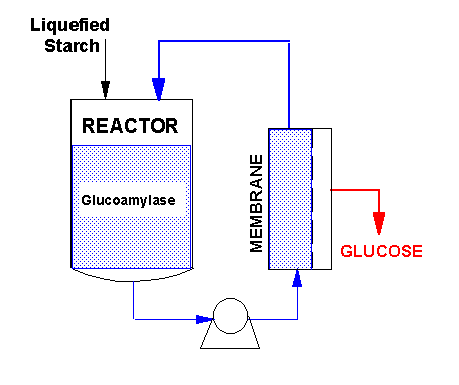
To overcome these limitations, University of Illinois biochemical engineers began a research project in 1986 to develop a "continuous membrane reactor" (CMR) for saccharification of starch. The CMR is based on the use of synthetic semipermeable membrane technology. These superfine filters can "reject" the starch and enzyme molecules while allowing the dextrose molecules to pass through.
As shown in the diagram, a reaction vessel is coupled in a closed-loop configuration to the appropriate membrane module. The feed (liquefied starch) is fed into the vessel, where it reacts with the glucoamylase, which breaks it down into dextrose molecules. The reaction mixture is continuously pumped through the membrane unit, which separates out the dextrose, while the enzymes and unreacted or partially reacted starch is recycled back to the vessel for further reaction. To bring the CMR concept to commercial reality, the project went through several stages of research and development. Initial studies were done in a bench-top dead-end stirred cell reactor with a total volume of 0.015 liters. Later, a larger system (0.5 liters) configured as a recycle-type membrane reactor was used to obtain a better understanding of the kinetics of the saccharification reaction, and to mathematically model the CMR to enable easier scale-up and optimization. By 1991, the CMR had been scaled up to 60 liters reaction capacity, using 8 sq.m. of membrane area, producing about 3-4 kg per hour of dextrose. After successful trials at the university's Agricultural Bioprocess Laboratory, a larger pilot plant was designed and fabricated for on-site testing at a large midwestern corn wet milling plant. It consisted of a 1500 liter reaction vessel and four two-element membrane housings (for a total membrane area of 30-45 sq. m.). The CMR operated successfully on site, and it was further optimized with inputs from membrane manufacturers, plant engineers and enzyme manufacturers. The chart below is a comparison of the CMR vs. the conventional batch process. Option 1 of the CMR is typical of the data obtained under the specified conditions during trials in the university's laboratory in 1991-92. In Option 2, the CMR is operated at a lower temperature, which should improve the CMR productivity, because the enzyme is more stable.
Benefits for a
biomass or corn refiner
Costs
Updated April 2000 by mcheryan@uiuc.edu | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Publications on this topic
Publications on this topic